By Emma Diamond
On December 2nd, the United Kingdom granted emergency authorization for the distribution of Pfizer’s Coronavirus Vaccine. On December 8th, only seven months after the beginning of the first clinical trials, they began distribution.
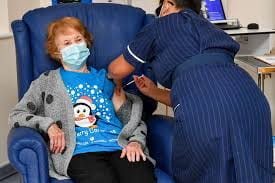
The first person to receive the Pfizer Coronavirus Vaccine after approval in the United Kingdom was a 90 year old woman named Margaret Keenan. Keenan explained to the media that she feels “privileged” to be the first to receive the vaccine. In addition, she pointed to her age and told the public, “If I could do this, well, so can you.” An 80 year old man named William Shakespeare was the second to receive the Pfizer vaccine. He sat near his grandson’s artwork while he was given the shot. A nurse named Joana Sloan became the first person to be vaccinated in Northern Ireland.
Two severe allergic reactions after being vaccinated with the Pfizer vaccine were reported by UK healthcare workers. Both patients who experienced these reactions have since recovered. After this report, people with a history of severe allergic reactions (those who have been told to carry an EpiPen) were advised to avoid the vaccine until healthcare workers and scientists can discover exactly what happened. Vaccines including the flu shot are known to cause allergic reactions in people who are allergic to certain preservatives and/or eggs. The Pfizer vaccine, however, does not contain any preservatives or eggs. In the United States clinical trials, which gave the vaccine to more than 20,000 people, a small portion, 1%, of patients experienced an allergic reaction. However, people with a history of severe allergic reactions were excluded from these trials. Although these new reports are concerning, most people will not experience these symptoms. In addition, symptoms of allergic reactions happen quickly after someone is vaccinated; it usually takes around fifteen to thirty minutes. Most health facilities that are distributing the shot are keeping patients there for around this much time after they are vaccinated anyway, so if someone were to have an allergic reaction, they would likely already be in a healthcare setting.
Healthcare workers in other countries, including the US and Poland, believe that the United Kingdom’s warning to all with severe allergies is excessive and unnecessary. Some believe that only people who have previously had reactions to vaccines should be advised not to take it. Others suggest that instead of issuing a warning for all people with allergies, experts should investigate what specific ingredients these two patients had a reaction to.
After hearing this news, American healthcare workers continue to be concerned about the American people’s willingness to take the vaccine. They worry that fear of getting an allergic reaction will prevent Americans from getting vaccinated. Polls show that about 60% of Americans report that they will take the vaccine if it is approved and available to them. Dr. Anthony Fauci says at least 75% would need to be vaccinated in order to achieve herd immunity. This means that the United States government is going to have to continue to convince 15% of the unwilling population that this vaccine is safe and worth getting.
On December 9th, the Food and Drug Administration committee met and approved Pfizer’s Coronavirus Vaccine for emergency use in the United States.
Hopefully the distribution process will happen quickly so we can return to our normal lives soon.
Sources:
https://www.cnn.com/2020/12/08/europe/uk-pfizer-biontech-covid-vaccination-intl/index.html
https://www.nature.com/articles/d41586-020-03441-8